The Role and Crisis of Opioids in Pain Therapy
Multifaceted Dimensions of Pain:
Opioids play a pivotal role in pain management because of their potent analgesic properties that make them indispensable for treatment of severe pain, especially in acute, cancer-related, and palliative care settings. However, opioid use is increasingly contributing to a global public health crisis, driven by their high potential for addiction, adverse side effects, and misuse. Beyond these concerns, research suggests that long term opioid use may weaken the immune system and interfere with the body’s natural healing processes.
Chronic pain, defined as daily pain lasting more than three months, can lead to cognitive dysfunction, sleep impairment, reduced physical function, and work absenteeism. Moreover, widespread reliance on opioids to manage this type of pain further escalated the global healthcare crisis.
Addressing the crisis of opioid overuse requires a comprehensive and multifaceted strategy. This includes development and implementation of alternative, non-opioid pain management therapies that minimize potential risks while maintaining effective pain relief.
Predicament of Pain Therapy
Multifaceted Dimensions of Pain:
Over millions of years, pain has evolved into a highly sophisticated adaptive mechanism that integrates multiple biological processes across both the peripheral and central nervous systems to protect and repair the body. Pain conditions are inherently multifaceted, driven by distinct underlying pathways and encompassing various dimensions.
Chronic pain, in particular, exemplifies this complexity. Rather than being a mere extension of acute pain, it is a multidimensional and dynamic interaction among biological and psychosocial factors, with each continuously influencing the other. This continuous interaction contributes to a range of manifestations, including sensory disturbances (such as allodynia and hyperalgesia), neuropathic features, and alterations in emotional and cognitive functioning.
Current Challenges:
Multimodal therapy has been shown to improve outcomes and is widely recommended as the standard of care in many therapeutic areas, including pain management. To address the complexities of various painful conditions, healthcare professionals often prescribe multiple analgesics, each with a distinct mode of action. This approach frequently requires multiple prescriptions (the use of multiple drugs), raising concerns about safety, efficacy, proper dosing, and overall costs. A potential solution to these challenges is the development of fixed-dose combination drug products.
However,differences in the drugs’ molecular structures can lead to variable gastrointestinal absorptions, ultimately impeding both multiple-drug regimens and fixed-dose combination products from achieving synchronized pharmacokinetics and dual-pathway targeting. These differences in bioavailability create challenges in developing effective multimodal therapies while maintaining an optimal balance between robust pain relief and acceptable tolerability.
Unmet Need
Effectively addressing the multifaceted nature of pain demands, innovative approaches. Developing advanced products that integrate multiple drugs to simultaneously target distinct pain pathways is vital for ensuring both robust efficacy and safety.
Xgene Innovation
Patentable New
Chemical Entity
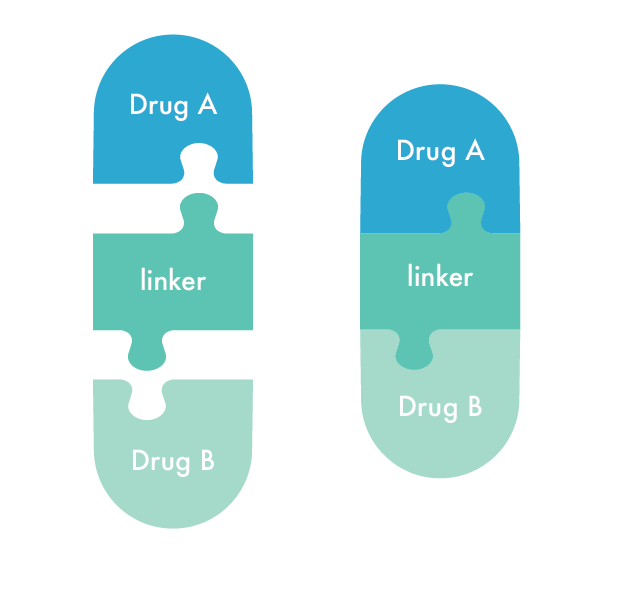
Through innovation, we have developed a new platform of designing dual-targeting prodrugs by chemically combining two drugs through conjugation. Within a single molecule, a dual-targeting prodrug combines the distinct actions of two drugs. For example, one component may be a cyclooxygenase (COX) inhibitor, addressing inflammation, pain, fever, and other physiological processes. Meanwhile, the other component targets the α2δ subunit of calcium channels, commonly used for treating neuropathic pain-related disorders, and central sensitization.
Upon absorption and cleavage in the bloodstream, the conjugated dual-targeting prodrug facilitates the coordinated release and synchronized pharmacokinetic profile of the parent drugs. This approach effectively targets multiple pain pathways simultaneously, while improving the overall pharmacokinetics and bioavailability. By optimizing these properties to enhance efficacy, conjugation also mitigates the potential side effects associated with the parent drugs. This innovative analgesic represents a breakthrough in pain therapy, offering significant advantages over traditional multi-drug regimens or fixed-dose combinations.
Xgene’s innovative platform provides a unique multimodal approach to address the complex multifaceted dimensions of pain therapies. By leveraging medications with mechanisms of action that complement or synergistically enhance each other, Xgene aims to improve treatment outcomes while potentially reducing the complexities and risks associated with the current standard of care.
Values
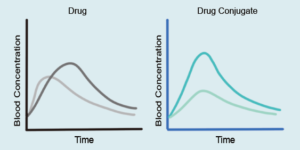
Synchronized PK Profiles between Drug A and Drug B
A reduction in GI and Central Nervous System side effects (headache, dizziness, sleepiness, confusion, etc.)
Expanding indications beyond
the parent drug
Driven by a patient-centric approach to R&D, our efforts begin by addressing difficult-to-treat, unmet patient needs through development of non-opioid, dual-targeting conjugated New Chemical Entities. These novel, patent-protected drugs have the potential to significantly reduce, if not eliminate, the need for opioids in managing diverse painful conditions. Leveraging the collective internal expertise of our staff and external key opinion leaders, Xgene is developing a unique class of new analgesics with particular emphasis on next-generation multi-modal approaches to pain treatment. Through science-driven strategies, we are progressing a comprehensive pip.
PRODUCT CANDIDATE
DISCOVERY
PRECLINICIAL
PHASE 1
PHASE 2
PHASE 3
RIGHTS
ACUTE PAIN
XG005
Oral Tablet
Dual-targeting prodrug conjugate of an NSAID and a gabapentinoid completed II/III trial following 505(b)1 FDA regulatory pathway in the US, demonstrating statistically significant and clinically meaningful analgesic efficacy and improved safety profile, in the acute post-surgical pain following bunionectomy.
Global
CHRONIC PAIN
XG005
Oral Tablet
Dual-targeting prodrug conjugate of an NSAID and a gabapentinoid completed phase II trial following Class 1 regulatory pathway in China, demonstrating statistically significant and clinically relevant results in the chronic pain due to osteoarthritis.
Global
CHRONIC PAIN
XG004
Topical Gel
Dual-targeting prodrug conjugate of an NSAID and a gabapentinoid with high transdermal penetration properties. A Phase Ib/IIa trial, including exploratory analgesic endpoints, in patients with Osteoarthritis of the knee has been completed in Australia with a positive analgesic signal and statistically significant improvement in sleep quality.
Global
CHRONIC PAIN, METASTATIC BONE PAIN
XG2002
Novel Target
TRPM8 channel blocker successfully completed phase I trial in Australia.
Global Excluding Japan
XG045
Patent-pending dual mechanism of action NCE
Global
XG046
Patent-pending dual mechanism of action NCE
Global
Regulatory status of XG005 drug conjugate
NMPA : Recognized it as Class 1 Innovative Drug
U.S. FDA: Designated as NCE with the 505(b)1 development pathway
Clinical development pathway has the potential for acceleration
NMPA directly allows simultaneous development of XG005 for two indications
XG005 entered directly from Phase 1 into first potentially pivotal trial in the US
Pipeline
ACUTE PAIN
XG005
Oral Tablet
Phase 3
Dual-targeting prodrug conjugate of an NSAID and a gabapentinoid completed II/III trial following 505(b)1 FDA regulatory pathway in the US, demonstrating statistically significant and clinically meaningful analgesic efficacy and improved safety profile, in the acute post-surgical pain following bunionectomy.
Global rights
CHRONIC PAIN
XG005
Oral Tablet
Phase 2
Dual-targeting prodrug conjugate of an NSAID and a gabapentinoid completed phase II trial following Class 1 regulatory pathway in China, demonstrating statistically significant and clinically relevant results in the chronic pain due to osteoarthritis.
Global rights
CHRONIC PAIN
XG004
Topical Gel
Phase 1b/2a
Dual-targeting prodrug conjugate of an NSAID and a gabapentinoid with high transdermal penetration properties. A Phase Ib/IIa trial, including exploratory analgesic endpoints, in patients with Osteoarthritis of the knee has been completed in Australia with a positive analgesic signal and statistically significant improvement in sleep quality.
Global rights
CHRONIC PAIN, METASTATIC BONE PAIN
XG2002
Novel Target
Discovery/Pre-clinical has been finished
TRPM8 channel blocker successfully completed phase I trial in Australia.
Global Excluding Japan rights
Pain Discovery Program
XG045
Discovery
Patent-pending dual mechanism of action NCE
Global rights
Pain Discovery Program
XG046
Discovery
Patent-pending dual mechanism of action NCE
Global rights
