October 25 ,2024
Xgene’s Osteoarthritis Study Accepted as Late-Breaking Abstract to Present at the American College of Rheumatology (ACR) Convergence 2024 Conference

December 12 ,2024
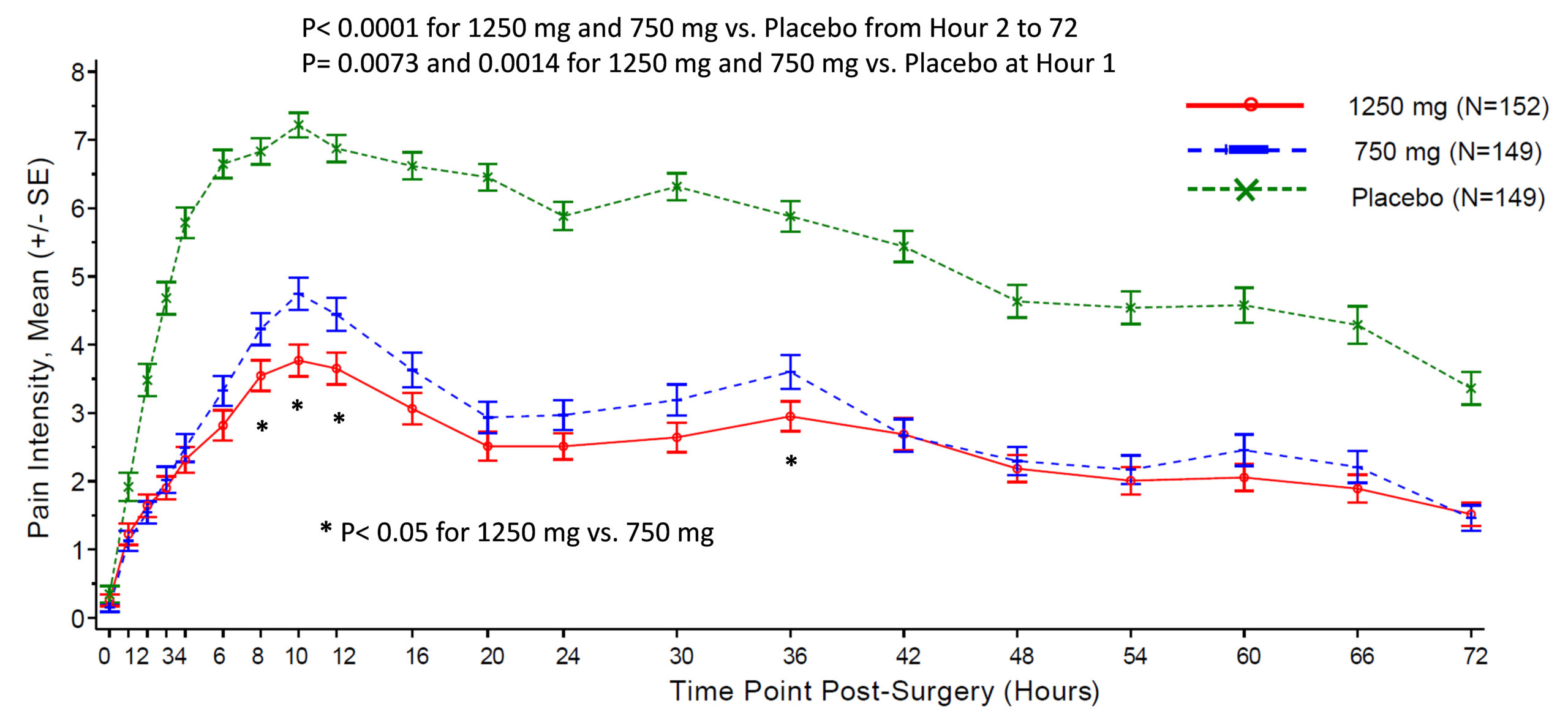
Xgene Pharmaceutical Announces Positive Topline Results from Late Phase Trial of XG005 Tablet for Post-surgical Pain

August 8,2024
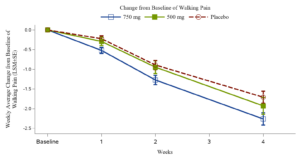
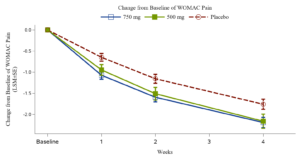
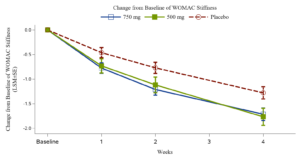
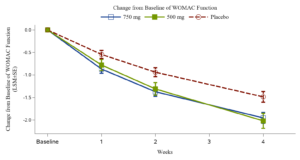
Xgene Pharmaceutical Announces Positive Topline Results from Phase 2b Trial of XG005 Tablet for Treatment the Symptoms of Osteoarthritis of the Knee

October 25 ,2024
Xgene’s Osteoarthritis Study Accepted as Late-Breaking Abstract to Present at the American College of Rheumatology (ACR) Convergence 2024 Conference

May 08,2024
Xgene Pharmaceutical Pty Ltd. Announced the Enrollment of the First Cohort in the Phase I clinical trial of XG2002 capsules, a novel selective TRPM8 channel blocker.

April 08,2024
Xgene Pharmaceutical Inc. announced achievement of the target patient enrollment in the phase II clinical trial of XG005 oral tablet in the treatment of pain associated with Osteoarthritis.

March 11,2024
Xgene Pharmaceutical Pty Ltd. Receives HREC’s approval to initiate a First-In-Human (FIH) trial of its innovative analgesic product XG2002

August 23,2023
Xgene Pharmaceutical Inc. Announces Dosing of First Patient in a Phase 2b/3 Trial of XG005 for the Treatment of Pain Following Bunionectomy Surgery.

April 26,2023
Xgene Pharmaceutical Announces Positive Top-Line Results from Phase 1b/2a Trial of Topical Gel XG004 for the Treatment of Osteoarthritis (OA) Pain of the Knee

November 04,2021
Xgene Pharmaceutical secures $40m in Series C

September 22,2021
Xgene Pharmaceuticals Co. Ltd.and RaQualia Pharma Inc.have entered into a licensing agreement for a novel TRPM8 blocker discovered by RaQualia Pharma

April 22,2020
NDA Application of XG005 will follow a 505(B)(1) regulatory pathway

May 4, 2019
XGene Pharmaceutical Closed the B Plus
Round Financing

October 25, 2018
XGene Pharmaceutical Closed US$20 Million B Round Financing